In the field of vein treatments, staying informed about the latest developments is crucial for both medical professionals and patients seeking effective and safe procedures. One significant aspect of this evolution is the role of the U.S. Food and Drug Administration (FDA) in approving and regulating vein treatment methods. In this blog, we’ll delve into the latest FDA approvals and regulations related to vein treatments, shedding light on what patients and practitioners can expect in this dynamic landscape.
The FDA’s Role in Vein Treatment Approvals:
The FDA plays a pivotal role in ensuring the safety and efficacy of medical devices and procedures, including those related to vein treatments. New technologies and innovations in the field of venous care continually undergo rigorous evaluation to receive FDA approval. This approval process involves comprehensive testing and clinical trials to verify the safety and effectiveness of vein treatment methods.
Recent FDA Approvals in Vein Treatments:
Minimally Invasive Procedures:
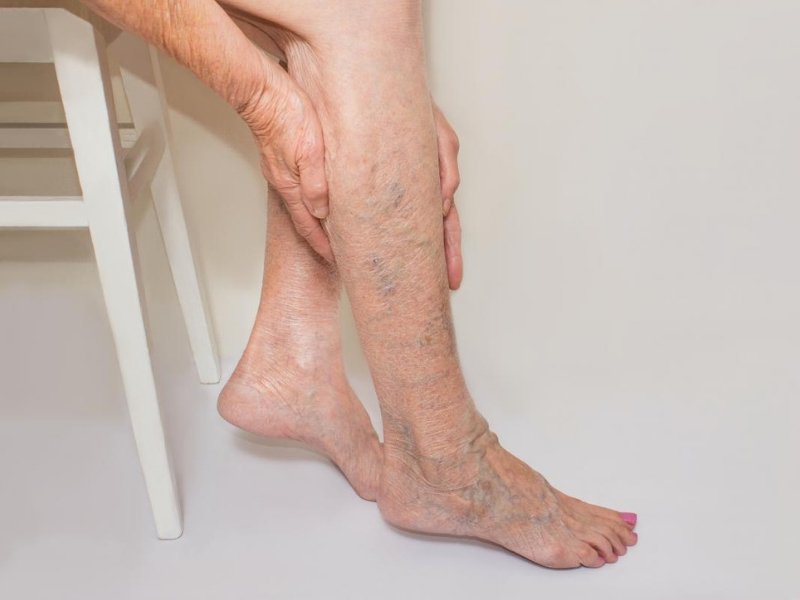
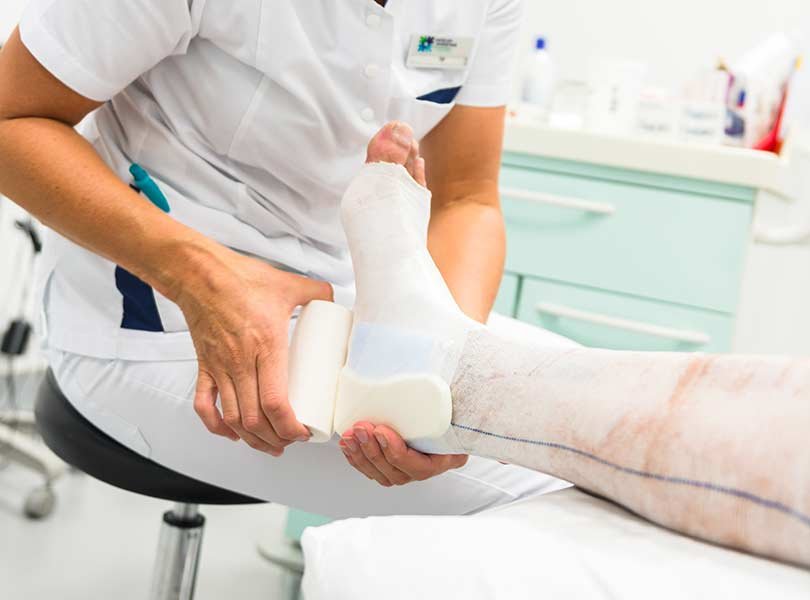
Over the past few years, the FDA has approved various minimally invasive procedures for vein treatments, such as endovenous laser therapy (EVLT) and radiofrequency ablation (RFA). These methods offer less invasive alternatives to traditional surgical procedures for treating varicose veins and have become increasingly popular due to their effectiveness and shorter recovery times.
Sclerotherapy Solutions:
The FDA has also approved new sclerotherapy solutions, which are used to treat spider veins and small varicose veins. These solutions are more effective and less painful, improving the patient experience during and after treatment.
Vena-Seal Closure System:
The Vena-Seal Closure System is an FDA-approved adhesive treatment for venous reflux disease. This system uses medical adhesive to seal the affected vein, providing a minimally invasive and highly effective treatment option for patients.
Regulations Ensuring Safety and Efficacy:
Apart from approvals for specific treatments, the FDA also enforces regulations to ensure the safety and effectiveness of vein treatments as a whole. These regulations are in place to protect the well-being of patients and maintain the integrity of the medical field. They encompass aspects like:
Clinical Trials:
Vein treatment methods must undergo rigorous clinical trials to demonstrate their safety and effectiveness. The results of these trials are carefully reviewed by the FDA before a treatment is approved.
Quality Control:
The FDA sets stringent quality control standards for the manufacturing and administration of vein treatment procedures and devices. This ensures that patients receive safe and reliable treatments.
Monitoring and Reporting:
Medical professionals are required to monitor patient outcomes and report any adverse events associated with vein treatments to the FDA. This continuous feedback loop is essential for maintaining patient safety.
Patient Education and Informed Consent: Regulations emphasize the importance of providing patients with comprehensive information about the treatment, its potential risks, and expected outcomes. Informed consent is a key aspect of the treatment process.
The Impact on Vein Treatment Practices:
The latest FDA approvals and regulations are reshaping vein treatment practices in several ways:
More Options:
Patients now have access to a broader range of safe and effective vein treatment options, including less invasive procedures that reduce downtime.
Higher Standards:
Medical professionals are held to higher standards when it comes to treatment quality, safety, and patient care.
Improved Patient Outcomes:
The emphasis on clinical trials and reporting ensures that vein treatments are continually refined and improved, leading to better patient outcomes.
Enhanced Safety:
Stricter regulations prioritize patient safety, reducing the risk of complications and adverse events.
What Patients Should Know:
Patients seeking vein treatments should be aware of the FDA’s role in ensuring the safety and effectiveness of these procedures. When considering a vein treatment, it’s essential to choose a qualified medical professional who adheres to FDA regulations and is well-informed about the latest FDA-approved treatments.
In conclusion, the FDA’s role in approving and regulating vein treatments is a critical factor in the evolution of this field. Patients and medical professionals can benefit from the latest FDA approvals, which provide safer and more effective treatment options for venous issues. By staying informed and working with reputable healthcare providers, patients can make well-informed decisions about their vein treatment options.